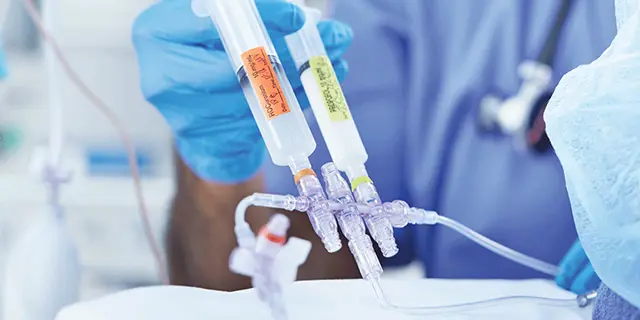
Specialty IV Consumables for Anesthesia
Infusion technology designed to help provide safe and efficient delivery of anesthetic medications
- Minimize the Potential for Bacterial Transfer
- Enhance Setup Efficiency
- Standardize Supply Chain
Help reduce the risk of anesthesiology-related infections while helping address inefficiencies
When it comes to anesthesia delivery, every detail matters. That’s why we’ve designed specialty products to help reduce the risk of anesthesiology-related infections while helping address inefficiencies in intraoperative environments.
From the infection control properties of our clinically-differentiated Clave™ needlefree connectors, to procedure-ready IV sets that let you streamline the delivery of care, we give you access to the tools you need to help safely and efficiently deliver anesthesia medications to your patients.
The Challenge
High levels of contamination in the anesthesia work environment point to the need for enhanced infection control technology
The administration of intravenous (IV) fluids is essential in anesthesia, but your typical operating room (OR) can be crowded and cluttered with equipment that may not be sterile.
Due to the necessity of frequent contact with potential sources of bacterial transfer, there’s a high probability of patient contamination during anesthesia administration.
Up to 32% of open IV stopcock sets may become contaminated in the OR.1
Up to 25% of patients with contaminated stopcocks may develop infections.1
Four of the most contaminated sources in the OR are the anesthesia keyboard and mouse, the anesthesia cart drawers, and the OR bed.2, 4
Help Minimize Infection Risks
Enhance patient safety and comply with CDC guidelines 9
Incorporate closed, self-sealing components featuring Clave IV connector technology
ICU Medical’s full line of needlefree stopcocks and manifolds feature clinically-differentiated Clave infection control technology, designed to minimize the risk of contamination by maintaining a closed system. These access ports are ideal for anesthesiology where simultaneous fluid delivery is critical.
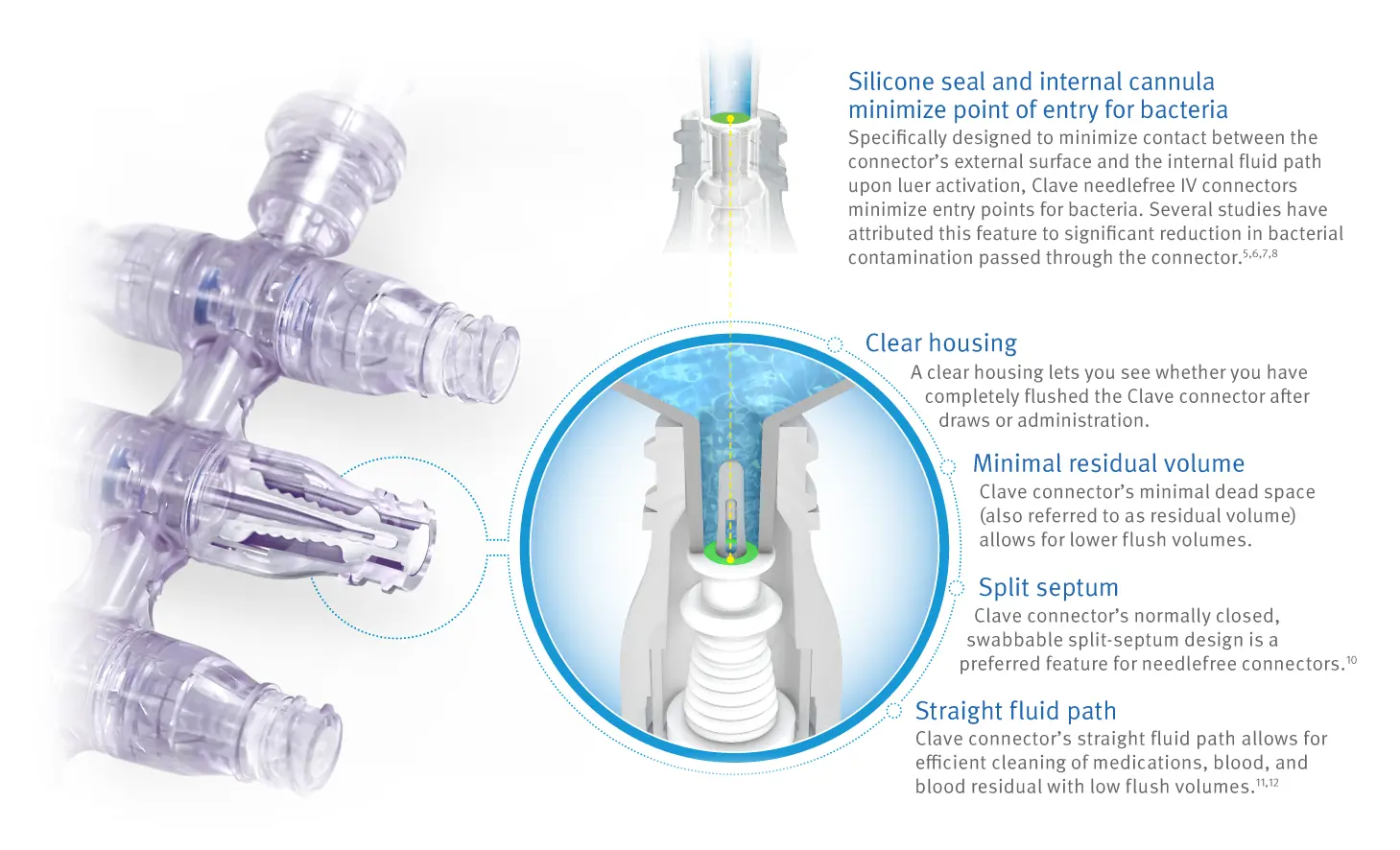
Clave technology has been proven to minimize bacterial transfer 5,6,7,8
In a peer-reviewed study comparing 20 different needlefree IV connectors, researchers reported ICU Medical’s connectors featuring Clave technology were shown to have a lower bacterial transfer rate than any of the other connectors tested.
Bacterial transfer rate comparison of needlefree connectors5
Clave connector technology has the lowest bacterial transfer rate of all connectors tested
Warning: Clave connectors may be incompatible with some male-luer connectors including prefilled glass syringes. To avoid damage to the Clave or syringes or male luers which may result in delays of medication administration and possible serious adverse events, users should confirm mating luers or syringes have an internal diameter range of 0.062” to 0.110”. Check the internal diameter of the male-luer connector of the mating syringe prior to using it to access the Clave. Products outside of these dimensional tolerances should not be used.
Enhance Setup Efficiency
Address IV anesthesia inefficiencies
Assembling multiple IV sets and components for anesthesia delivery is not only complex, it requires extra manipulation, wastes time, and may lead to delays in care or an increased risk of contamination. ICU Medical's broad portfolio of procedure-ready IV sets for anesthesia can let you standardize processes and minimize setup time.
Anesthesiologists spend up to 29% of their time manipulating IV components13
Traditional anesthesia delivery methods requires the assembly of multiple IV sets and components which may increase packaging waste and clutter.
"Anesthesia workspaces were observed to become cluttered with unwanted sterile packaging, occasionally obscuring a clear view of more critical items like drug syringes or airway supplies." — Fraind et al.13
Get procedure-ready IV sets designed to meet your specific needs
Our broad portfolio of IV sets can help improve the efficiency of your anesthesia IV drug delivery practices by letting you choose patient-ready sets designed to match your specific need, avoiding the burdensome assembly of multiple components at the bedside. When you consolidate inventory of different set SKUs, you free up shelf space, save valuable time spent searching for the set you need, and reduce packaging waste and clutter.
Product Information
Related Products
Product Inquiry
Please enter your details into the following form.
References
-
Loftus R, Koff M, Burchman C, Schwartzman J, Thorum V, Read M, Wood T, Beach M. Transmission of pathogenic bacterial organisms in the anesthesia work area. Anesthesiology 2008;109: 399-407
-
Link T, Kleiner C, Mancuso MP, Dziadkowiec O, Halverson-Carpenter K. Determining high touch areas in the operating room with levels of contamination. Am J Infect Control. 2016;44: 1350-1355
-
Bibble C, Shah J. Quantification of anesthesia providers’ hand hygiene in a busy metropolitan operating room: what would Semmelweis think? AM J Infect Control. 2012;40: 756-759.
-
Franklin E, Stein L. Human Factors Analysis of Infection Prevention Practices in the Anesthesia Work Environment. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2017;61: 639-642.
-
Ryder, M., DeLancey-Pulcini, E., Parker, A., & James, G. (2023). Bacterial transfer and biofilm formation in needleless connectors in a clinically simulated in vitro catheter model. Infection Control & Hospital Epidemiology, 1-9. doi:10.1017/ice.2023.60.
-
JD Brown, HA Moss, TSJ Elliot. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997.; 36: 181-189.
-
Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med. 2008; 36: 2558-2561.
-
Bouza E, Munoz P, Lopez-Rodriguez J, et al. A needleless closed system device (Clave protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003; 54:279-287.
-
The Centers for Disease Control and Prevention (CDC). Guidelines for the Prevention of Intravascular Catheter-Related Infections. 2011.
-
Guidelines for the Prevention of Intravascular Catheter-Related Bloodstream Infections, 2011 (Updated Recommendations July 2017)
-
Data on file at ICU Medical. Low Volume Flush Characteristics of Unique Needlefree Connections M1-1223 Rev. 1.
-
Breznock EM, DVM, PhD, Diplomate ACVS, Sylvia CJ, DVM, MS, BioSurg, Inc. The in vivo evaluation of the flushing efficiency of different designs of clear needlefree connectors. March 2011.
-
Fraind D, Slagle J. Tubbesing V, Hughes S., Weinger M. Reengineering IV drug and fluid administration processes in the OR. Anesthesiology 2002;97: 139-147.