Tego™
Needlefree Hemodialysis Connector
Designed to help protect your patients’ catheters from contamination and increased risk of catheter-related bloodstream infections (CRBSIs) during hemodialysis and apheresis applications
-
Use of Tego was shown to be associated with a 10-12% reduction in CRBSIs.1
-
Straight fluid path accommodates flow rates of > 600 mL/min.
-
Validated for continuous patient use for up to 7 days.
Help protect your patients’ hemodialysis catheters from contamination and minimize the risk of CRBSIs
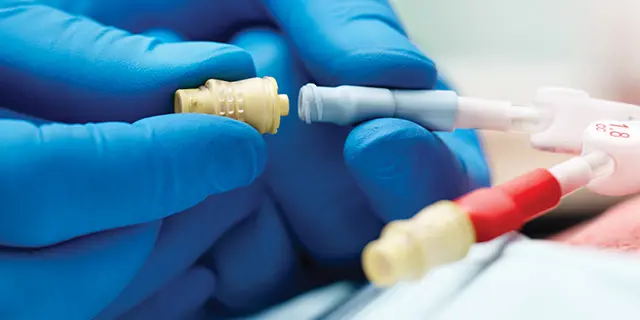
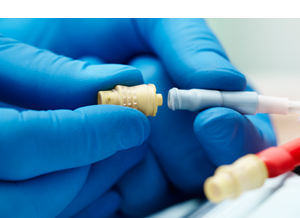
The Tego needlefree hemodialysis connector creates a closed system when attached to the hub of a catheter to minimize contamination and help reduce the risk of CRBSIs. Tego’s saline flush option is designed to help reduce the risk of heparin-induced thrombocytopenia (HIT) and helps you minimize overall hemodialysis costs by reducing heparin use.2-3
Functional Attributes
- Straight fluid path accommodates flow rates of greater than 600 mL/min
- Remains in place during the entire hemodialysis treatment period
- Validated for continuous patient use for up to 7 days
- Silicone seal remains closed when not activated, closing the fluid path
Clinical Advantages
- Use of Tego was shown to be associated with a 10-12% reduction in CRBSIs1
- Tego is a needle-free capping device which closes the end of a hemodialysis catheter, creating a mechanically closed system, prohibiting microbial ingress when attached to the hub of a hemodialysis catheter. Tego will permit access to the catheter without the use of needles, and therefore passively aid in the reduction of needlestick injuries.
- Tego's saline flush option helps to reduce risks and costs associated with heparin use.
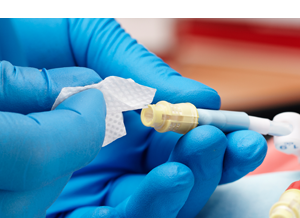
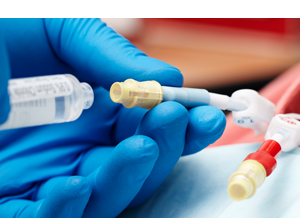
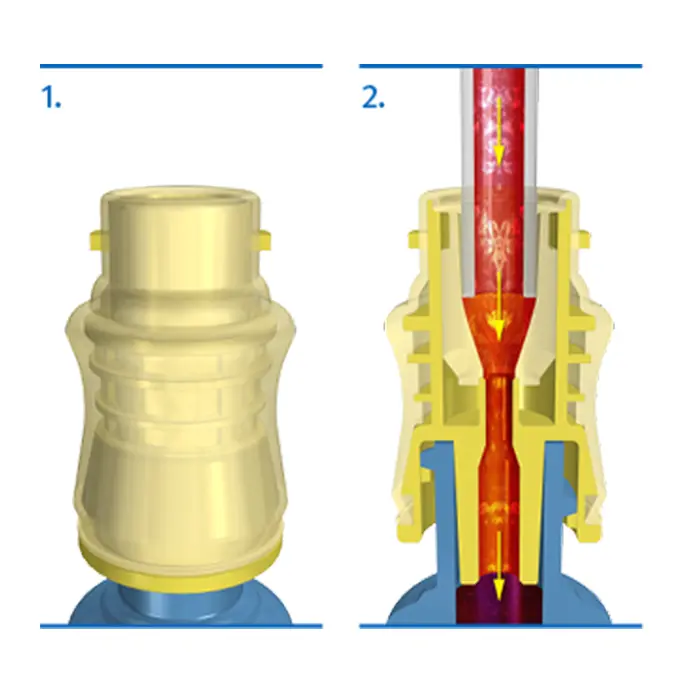
Accessing the Fluid Path:
- When Tego is not being accessed, the silicone seal forms a swabbable barrier to bacterial ingress.
- Attaching a dialysis bloodline to Tego activates the straight internal fluid path and creates a mechanically closed system, prohibiting microbial ingress.
Related Products
Product Inquiry
Please enter your details into the following form.
References
-
Brunelli SM, Njord L, Hunt AE, Sibbel SP. Use of the Tego needlefree connector is associated with reduced incidence of catheter-related bloodstream infections in hemodialysis patients. Int J Nephrol Renovasc Dis. 2014; 7: 131–139.
-
Farthing, C et al. Tego connectors reduce heparin use without affecting blood flow rate compared to traditional central venous catheter locks. American Nephrology Nurses Association 43rd National Symposium, Orlando, FL, Apr 29-May 2, 2012.
-
Krishnan M et al. Tego connectors reduce heparin use without affecting blood flow rate compared to traditional central venous catheter locks. American Society of Nephrology Kidney Week. Philadelphia, PA, Nov 8-11, 2011.