ViaValve™
Safety IV Catheter
Peripheral IV catheter that provides blood control and full encapsulated needle protection
Help Enhance Patient Comfort with V-Point Technology While Minimizing Blood Exposure
ViaValve Safety IV Catheters are designed with V-point technology to help minimize pain and venous trauma during insertion.
ViaValve Safety IV Catheters also provide blood control to help reduce the risk of blood exposure and contamination.
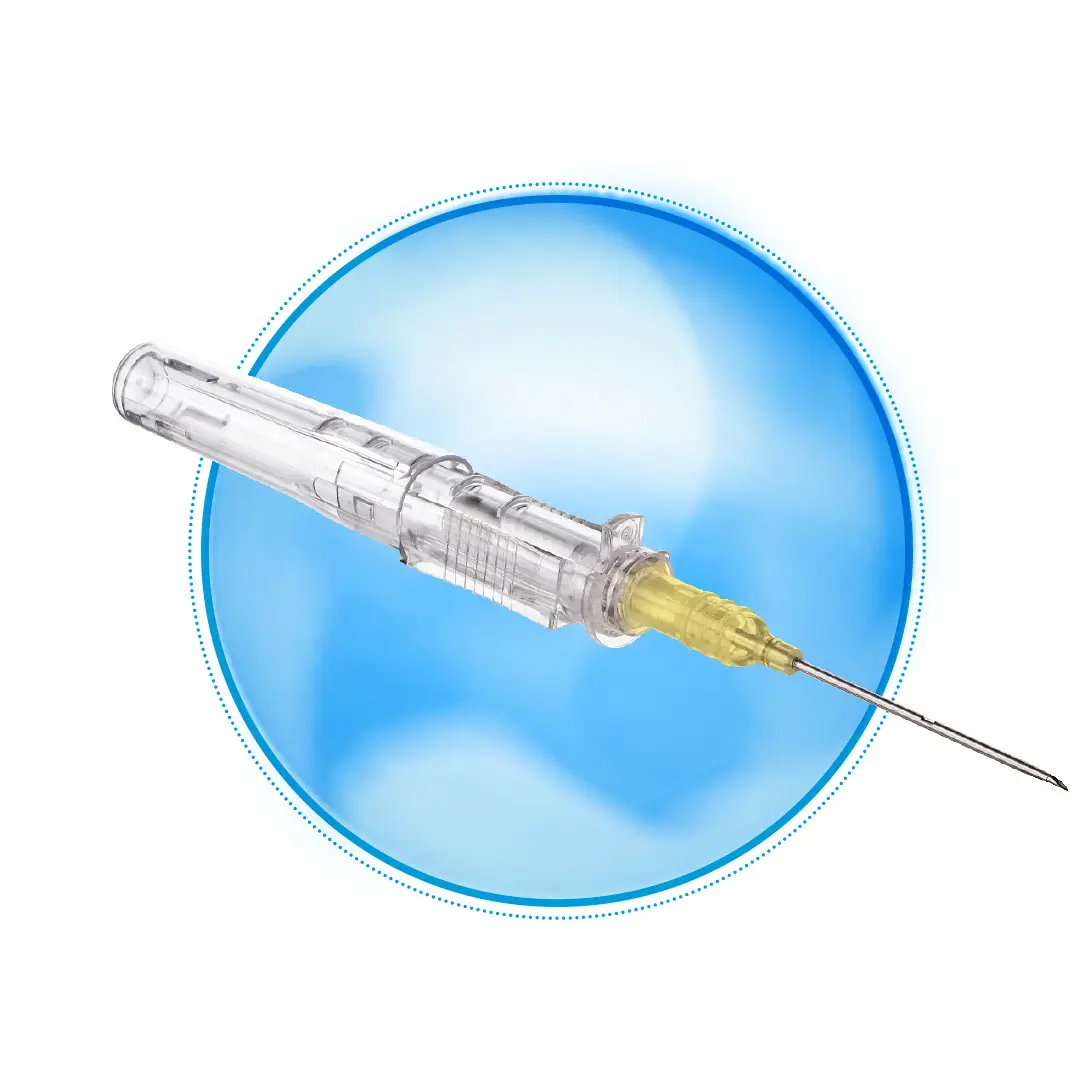
Avoid blood leakage during catheter insertion and setup with an integrated blood-containment valve.
Avoid accidental needlesticks and needle exposure with needle encapsulation.
Help ensure the catheter is properly in the vessel on the first stick with flash technology and Flash-Vue™ early flash indicator on gauges 20, 22, and 24.
Product Details
ViaValve™ Safety IV Catheter
Features Flash-Vue™ notched needle for early flash detection.
ViaValve™ Winged Safety IV Catheters
Features Flash-Vue™ notched needle for early flash detection.
Related products
Product inquiry
Please enter your details into the following form.
