Clave™
Clinically preferred needlefree IV connector technology
- Help reduce infection risks
- Standardize clinical protocols
- Optimize the supply of IV consumables
Choose clinically differentiated infection control technology to help enhance safety and efficiency
When you choose ICU Medical needlefree IV sets and connectors with proprietary Clave™ technology, you get an effective barrier against bacterial transfer and colonization designed to help reduce the risk of bloodstream infections.
And since the same clinical protocol is used throughout the hospital, you can standardize on a single connector technology wherever care is given, letting you minimize clinical training and in-servicing and provide enhanced patient safety throughout your facilities while optimizing your supply chain.
Minimize bacterial transfer and colonization with Clave needlefree connector technology
Intravenous therapy is essential to patient care, but accessing your patient’s bloodstream may increase the risk of infection. Clinically differentiated needlefree IV connector technology can be an important element in your efforts to help minimize the risk of bloodstream infections.
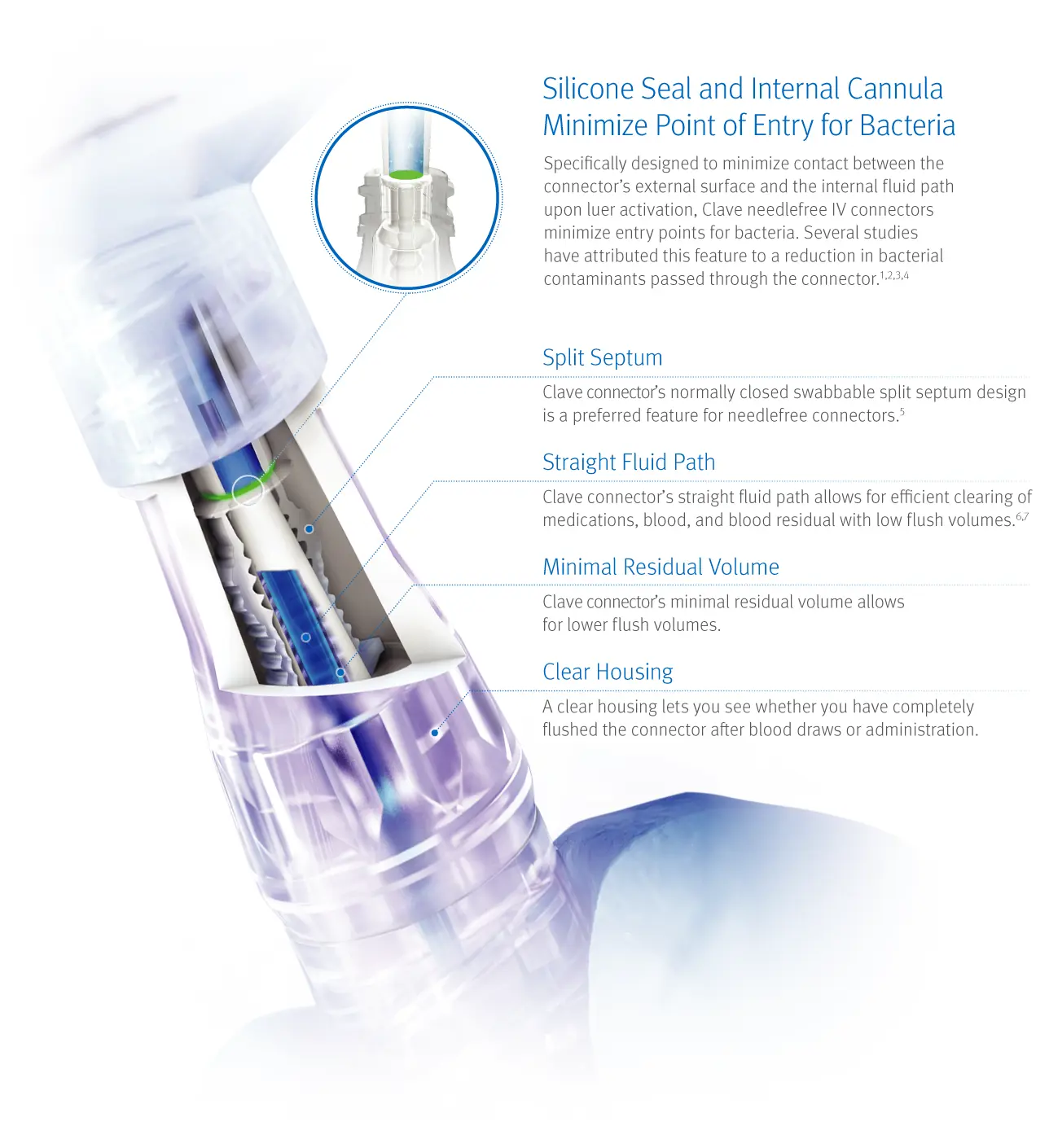
Enhance patient safety with one innovative technology in the DNA of every connector
Each of ICU Medical’s needlefree connectors utilizes the same Clave infection control technology, designed to enhance patient safety. Clave connectors straight fluid path and minimal residual volume help maximize the effectiveness of every flush.
MicroClave™ Clear
Neutral Displacement Connector
- Clear housing to visualize connector flushing
- Use on all vascular catheters
- Also available with blue tint for enhanced line identification
Clave™ Neutron™
Needlefree Neutral Displacement Connector
- Helps reduce multiple causes of catheter reflux
- Helps reduce catheter occlusions by as much as 50%8
NanoClave™
Manifolds and Stopcocks
- Minimize flush volumes
- Multiple applications, including multiport manifolds and stopcocks
Clave connectors help minimize entry points for bacteria and maximize the effectiveness of each flush
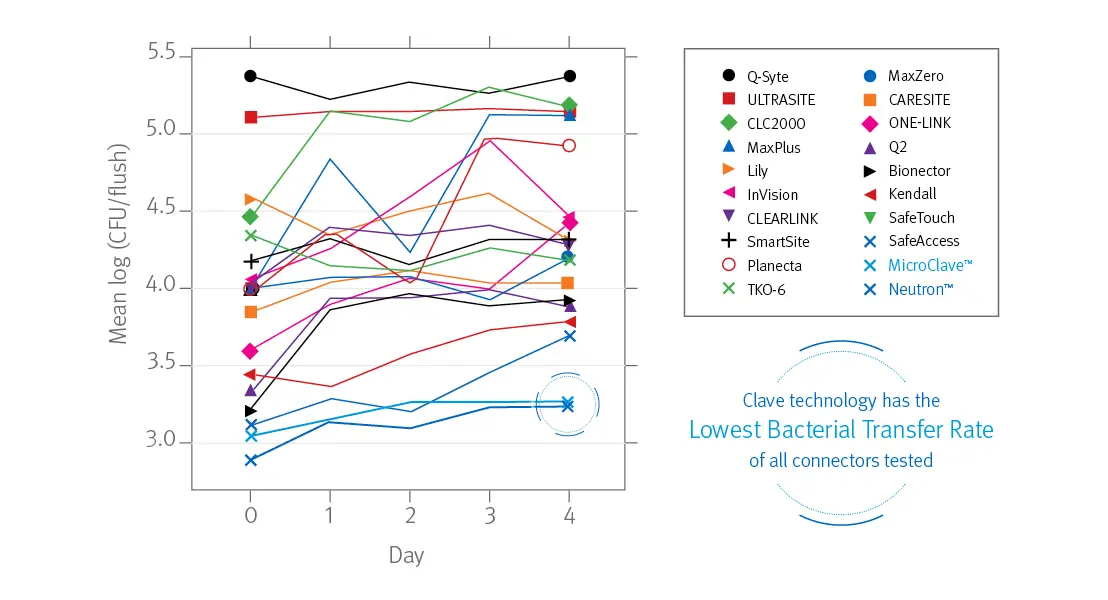
In a peer reviewed comprehensive study comparing 20 different needlefree IV connectors, researchers reported ICU Medical’s connectors featuring Clave technology were “shown to have a significantly lower bacterial transfer rate than any of the other connectors tested.”1
Bacterial transfer rate comparison of needlefree connectors
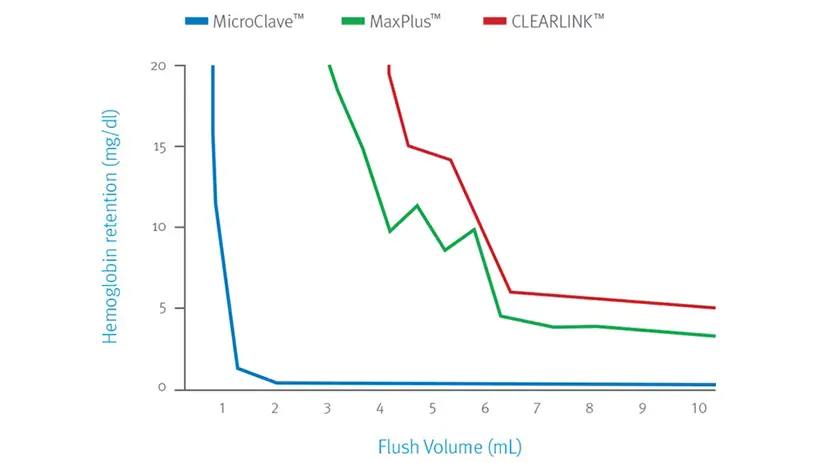
Efficiently clear the connector with low flush volumes
ICU Medical’s Clave technology outperforms the BD MaxPlus™ and Baxter Clearlink™ connectors as determined by the total flush volume needed to clear the connectors of residual blood elements.7
Warning: Clave connectors may be incompatible with some male-luer connectors including prefilled glass syringes. To avoid damage to the Clave or syringes or male luers which may result in delays of medication administration and possible serious adverse events, users should confirm mating luers or syringes have an internal diameter range of 0.062” to 0.110”. Check the internal diameter of the male-luer connector of the mating syringe prior to using it to access the Clave. Products outside of these dimensional tolerances should not be used.
MicroClave™
The clear choice to help reduce bloodstream infection risk and visualize connector flushing
MicroClave connectors combine clinically differentiated Clave technology with a clear housing to help you visualize connector flushing after blood draws or administration while providing an effective microbial barrier against bacteria transfer and contamination. Ideal for a wide range of clinical applications and patient populations, MicroClave is the optimal facility-wide needlefree IV connector.
MicroClave connectors are available with blue-tinted housing for enhanced line identification
Clave™ Neutron™
Needlefree Neutral Displacement Connector
Unique technology that helps reduce reflux and maintain catheter patency
Maintaining catheter patency and minimizing occlusions can be important steps in your efforts to enhance patient safety and reduce costs. Clave Neutron connector combines clinically differentiated Clave infection control technology with a proprietary bidirectional silicone valve and bellows feature to help reduce reflux.10 Clave Neutron connector helps maintain catheter patency during times when traditional connectors have been shown to occlude most often.
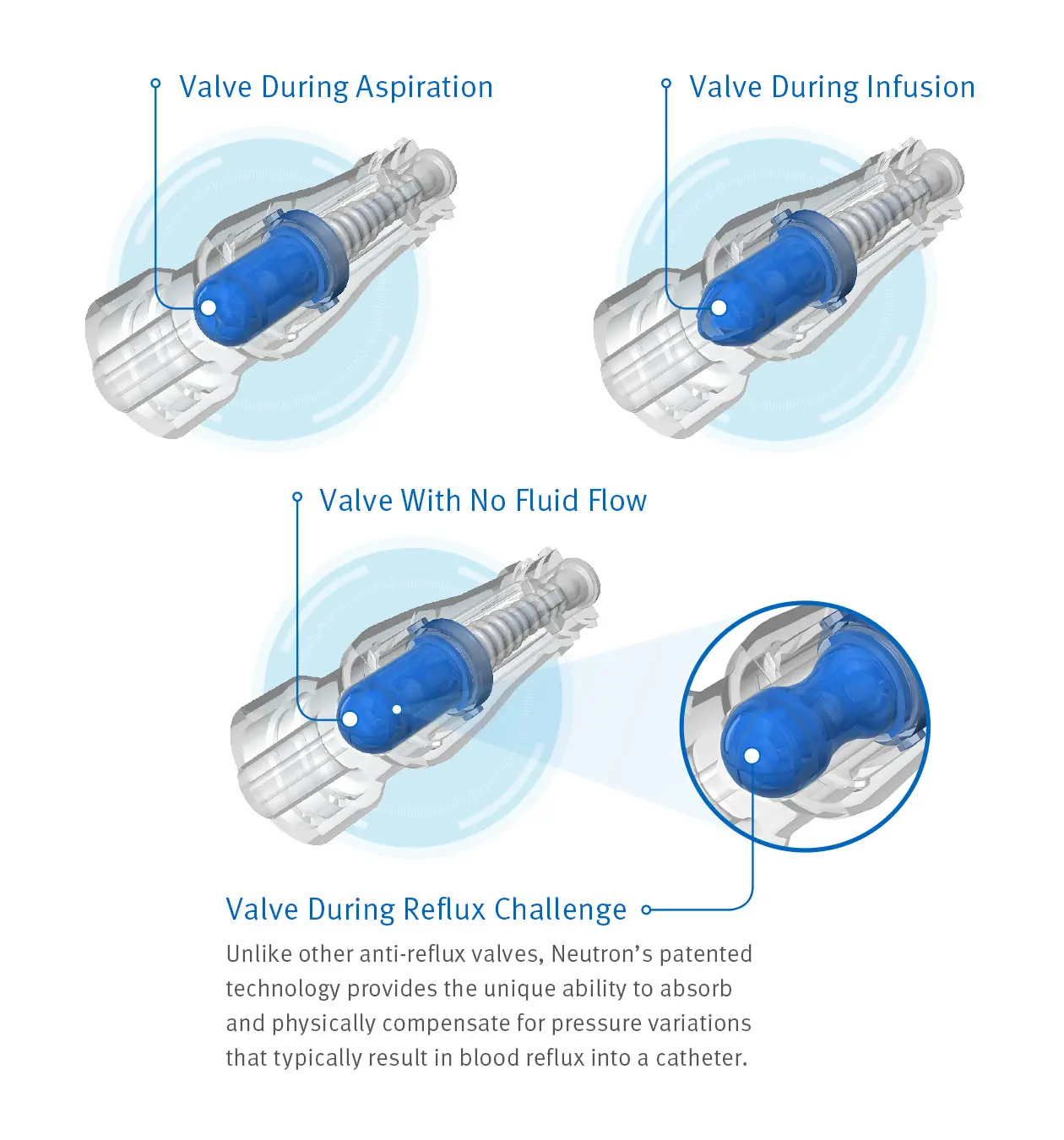
Clave Neutron connector may help you reduce catheter occlusions by 50%8
NanoClave™ Manifolds and Stopcocks
Optimize fluid delivery while helping protect against CRBSIs
Help reduce the risk of bloodstream infections and visualize connector flushing with NanoClave connector featuring a mechanically closed system that prohibits microbial ingress and provides a safe and effective microbial barrier, while a clear housing lets you visualize the internal fluid path when flushing.
Incorporating clinically-differentiated Clave technology at every connection
Stopcock and manifold ports integrate Clave infection control technology to maintain a closed system and enhance patient safety.
Optimize the supply of your essential IV consumables
Standardizing on ICU Medical IV consumables gives you best-in-class Clave technology and access to our full portfolio of components to optimize your supply chain across dedicated and non-dedicated sets and the broadest offering of off-the-shelf IV sets as well as the broadest offering of off-the-shelf IV sets tailored to a range of clinical needs.
Reduce SKUs with procedure-ready sets designed to meet your specific needs
Choose from our broad portfolio to meet your specific clinical need, letting you avoid ordering multiple components while maximizing shelf space and reducing packaging waste.
Related Products
Product Inquiry
Please enter your details into the following form.
References
- Ryder, M., DeLancey-Pulcini, E., Parker, A., & James, G. (2023). Bacterial transfer and biofilm formation in needleless connectors in a clinically simulated in vitro catheter model. Infection Control & Hospital Epidemiology, 1-9. doi:10.1017/ice.2023.60
- JD Brown, HA Moss, TSJ Elliott. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997.; 36:181-189.
- Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med 2008;36: 2558–2561.
- Bouza E, Munoz P, Lopez-Rodriguez J, et al. A needleless closed system device (Clave™) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003; 54:279-287.
- Guidelines for the Prevention of Intravascular Catheter-Related Bloodstream Infections, 2011 (Updated Recommendations July 2017)
- Data on file at ICU Medical. Low Volume Flush Characteristics of Unique Needlefree Connectors M1-1223 Rev. 1.
- Breznock EM, DVM, PhD, Diplomate ACVS, Sylvia CJ, DVM, MS, BioSurg, Inc. The in vivo evaluation of the flushing efficiency of different designs of clear needlefree connectors, March 2011.
- Observational In-Vivo Evaluation of the Neutron™ Needlefree Catheter Patency Device and its Effects on Catheter Occlusions in a Home Care Setting, 2011.
- Dayna Holt, MSN, RN, CRNI, CPN, VA-BC, Stephanie Lawrence, RN, BSN. The Influence of a Novel Needleless Valve on Central Venous Catheter Occlusions in Pediatric Patients. Journal of the Association For Vascular Access, Dec. 2015.
- Star Watts BSN RN CRNI VA-BC, Clinical Effect of the Neutron™ Needlefree Catheter Patency Device in Reducing PICC Line Occlusions and Cath-Flo™ Usage in a Teaching Hospital.