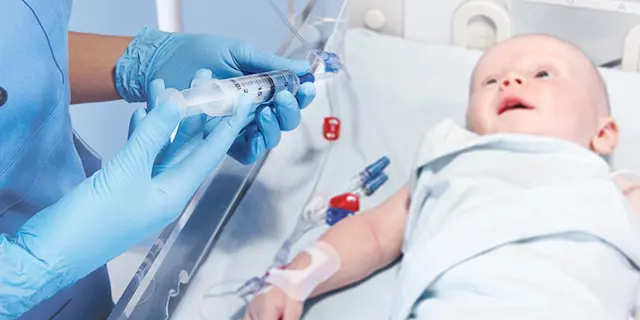
IV Consumables for the NICU and PICU
Specialty infusion technology for your most delicate patients.
Clave™ IV Connector Technology
Help minimize infection risks with manifolds and stopcocks that incorporate clinically-differentiated Clave connector technology
Specialized NICU/PICU IV Sets
Save time while helping reduce the risk of infection with closed IV medication sets for syringe pump delivery
SuperCath®5 26G Safety IV Catheter
Help deliver safe and effective care with the smallest-gauge straight IV catheter, helping you access small, difficult vessels
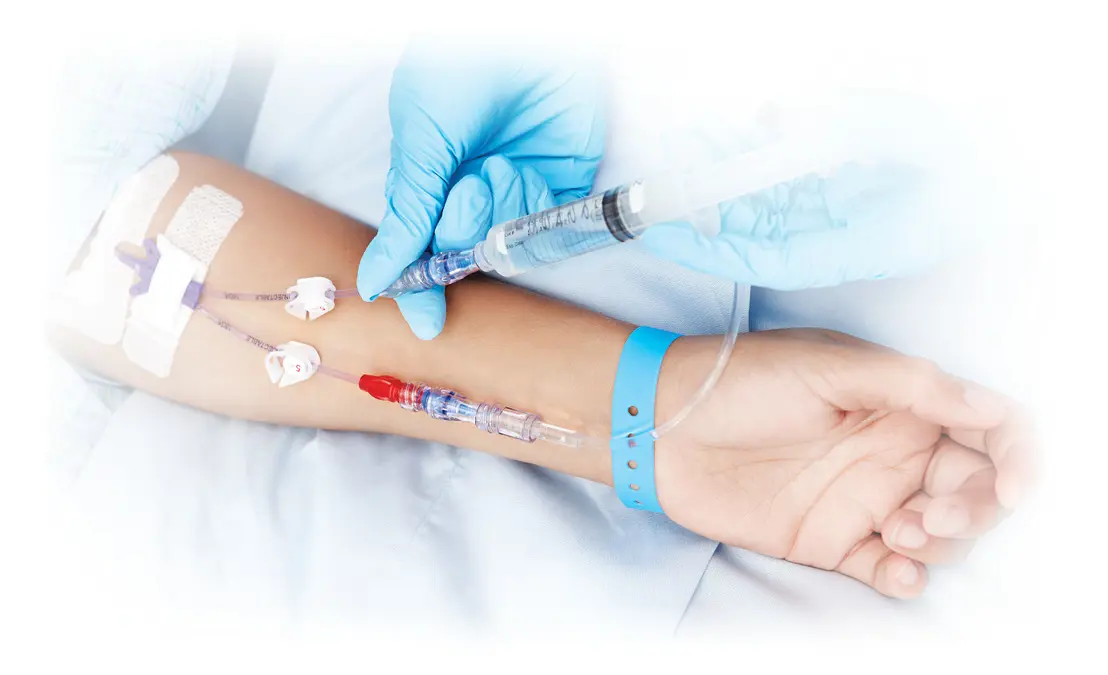
Special Patients Require Specialized Technology
Help minimize infection risks for your most delicate patients with clinically-differentiated Clave technology
Intravenous therapy is essential to patient care, but accessing your patient’s bloodstream may increase the risk of infection. Clinically-differentiated Clave needlefree IV connector technology can be an important element in your efforts to help minimize the risk of bloodstream infections.
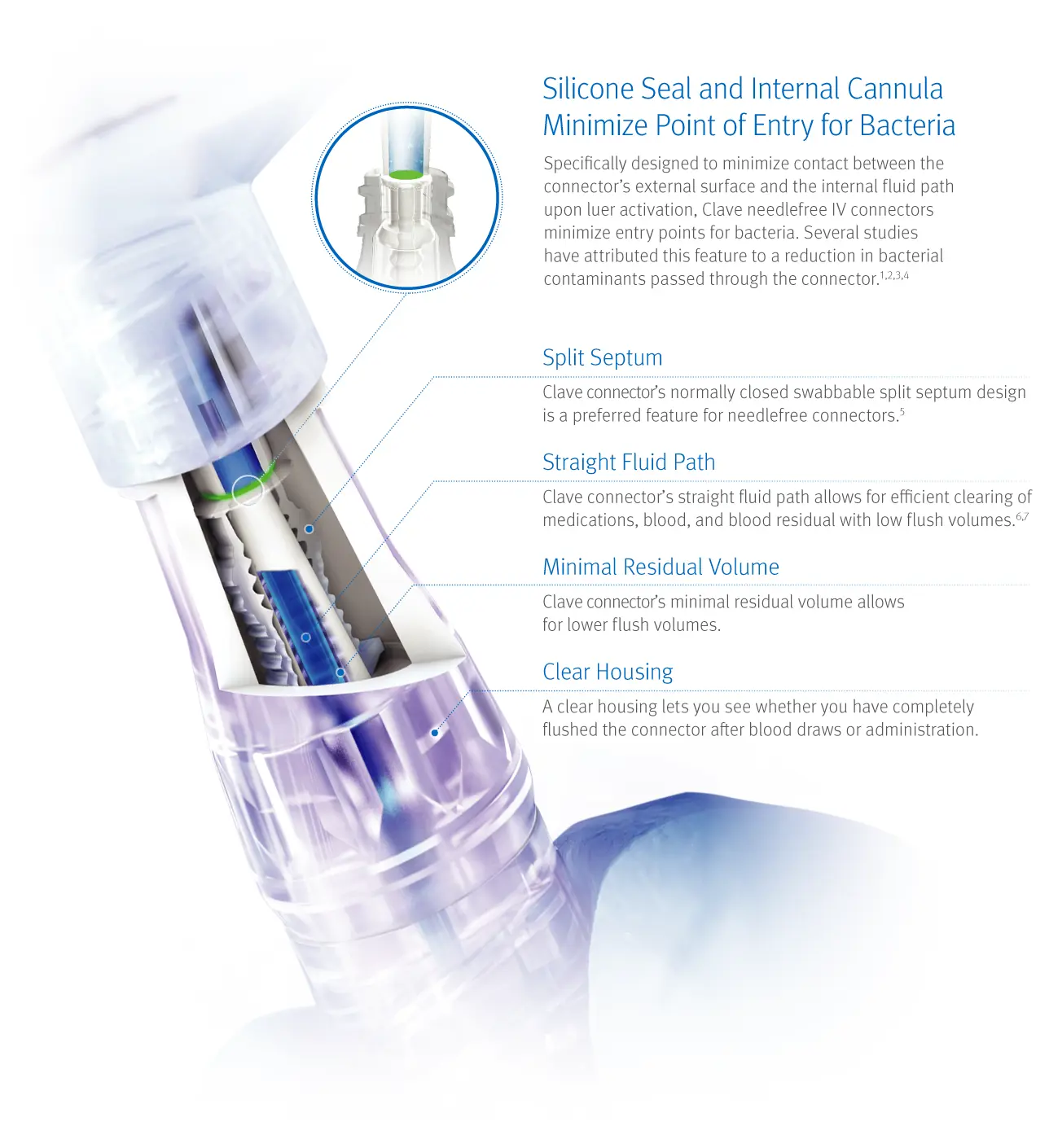
Clave Needlefree Infection Control Technology
Clave connector helps minimize entry points for bacteria and maximize the effectiveness of each flush
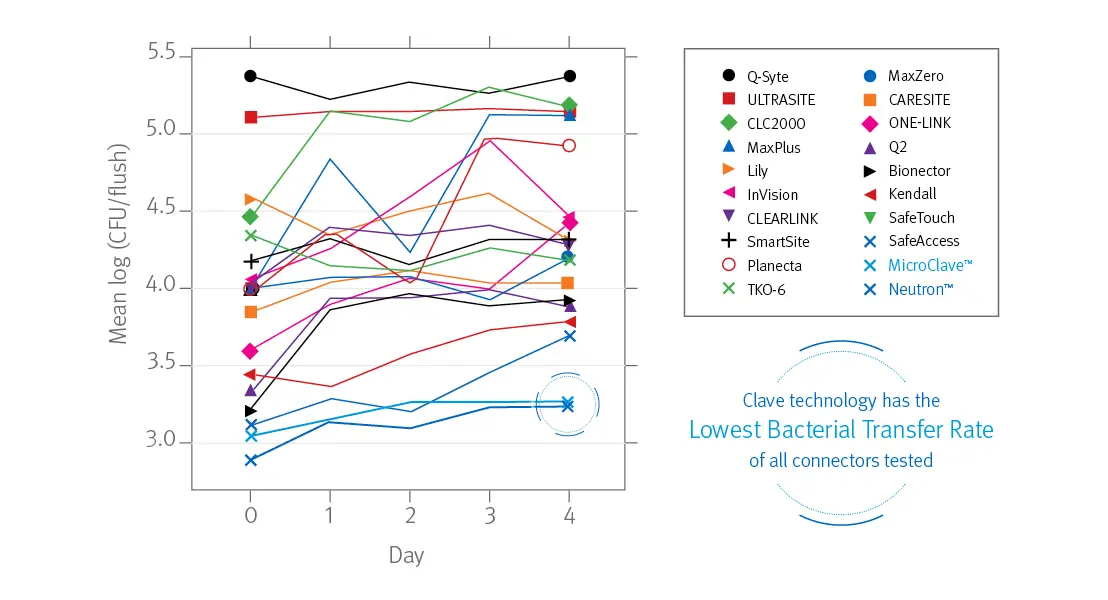
In a peer reviewed study comparing 20 different needlefree IV connectors researchers reported ICU Medical’s connectors featuring Clave technology were “shown to have a significantly lower bacterial transfer rate than any of the other connectors tested.”1
Bacterial transfer rate comparison of needlefree connectors
Warning: Clave connectors may be incompatible with some male-luer connectors including prefilled glass syringes. To avoid damage to the Clave or syringes or male luers which may result in delays of medication administration and possible serious adverse events, users should confirm mating luers or syringes have an internal diameter range of 0.062” to 0.110”. Check the internal diameter of the male-luer connector of the mating syringe prior to using it to access the Clave. Products outside of these dimensional tolerances should not be used.
The following are trademarks of their respective owners: Q-Syte™ (Becton, Dickinson and Company); MaxPlus™, SmartSite®, and MaxZero™ (CareFusion 303, INC.); ULTRASITE™, InVision™, and CARESITE™, (B. Braun Medical Inc.); TKO™-6 (Nexus Medical, LLC); Q2® (Quest Medical, Inc.); Lily™ (LILY Medical Corporation); CLEARLINK™ and ONE-LINK™ (Baxter International Inc.); Bionector™ (Vygon Corporation); Kendall™ (KPR U.S., LLC); SafeTouch™ (Nipro Corporation); Planecta™ (JMS CO, LTD)
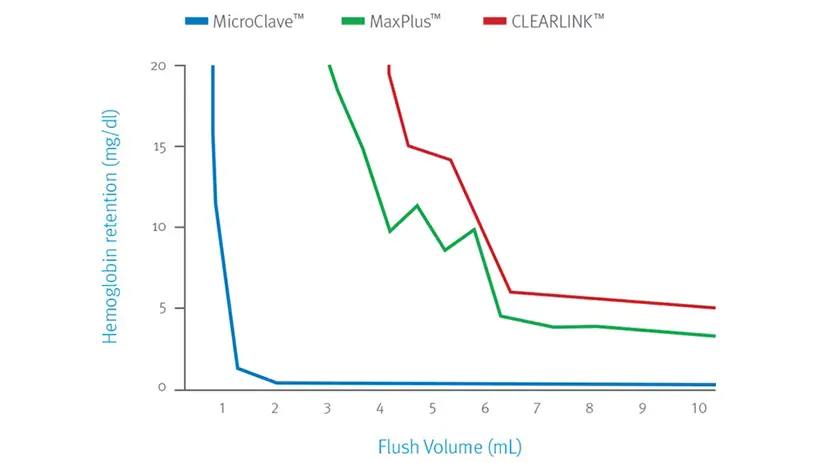
Efficiently clear the connector with low flush volumes
ICU Medical’s Clave technology outperforms BD MaxPlus™ and Baxter Clearlink™ connectors as determined by the total flush volume needed to clear the connectors of residual blood elements.6
NanoClave™ Manifolds and Stopcocks
Optimize fluid delivery with needlefree manifolds and stopcocks while helping protect against CRBSIs
Accessing your patient’s IV line through the hub of an open stopcock or manifold may increase the risk of bacterial contamination. Using manifolds and stopcocks with Clave needlefree IV connector technology can help efforts to minimize infection risks by maintaining a closed system and minimizing the risk of contamination.4
These access ports are ideal for anesthesiology, oncology, and critical care, where simultaneous fluid delivery is critical.

Specialized NICU/PICU IV Sets
Save time and money while helping reduce infection risks with customizable, closed IV medication sets
Manipulating traditional syringe pump tubing may increase the risk of medication errors and bacterial contamination.
Traditional, open-ended syringe pump administration sets require the manipulation of tubing, connectors, and flush devices, which adds to nursing setup time and may contribute to an increased risk of bloodstream infections and medication errors.8
Using ICU Medical’s closed IV medication sets during administration can help your efforts to minimize infection risks and improve medication safety.8
By incorporating Clave technology with dual one-way valve security, these fully customizable sets eliminate the need to connect and disconnect flush devices after medication delivery and remain completely closed throughout the entire drug delivery process.
Deliver medication and flush the line without ever opening the system

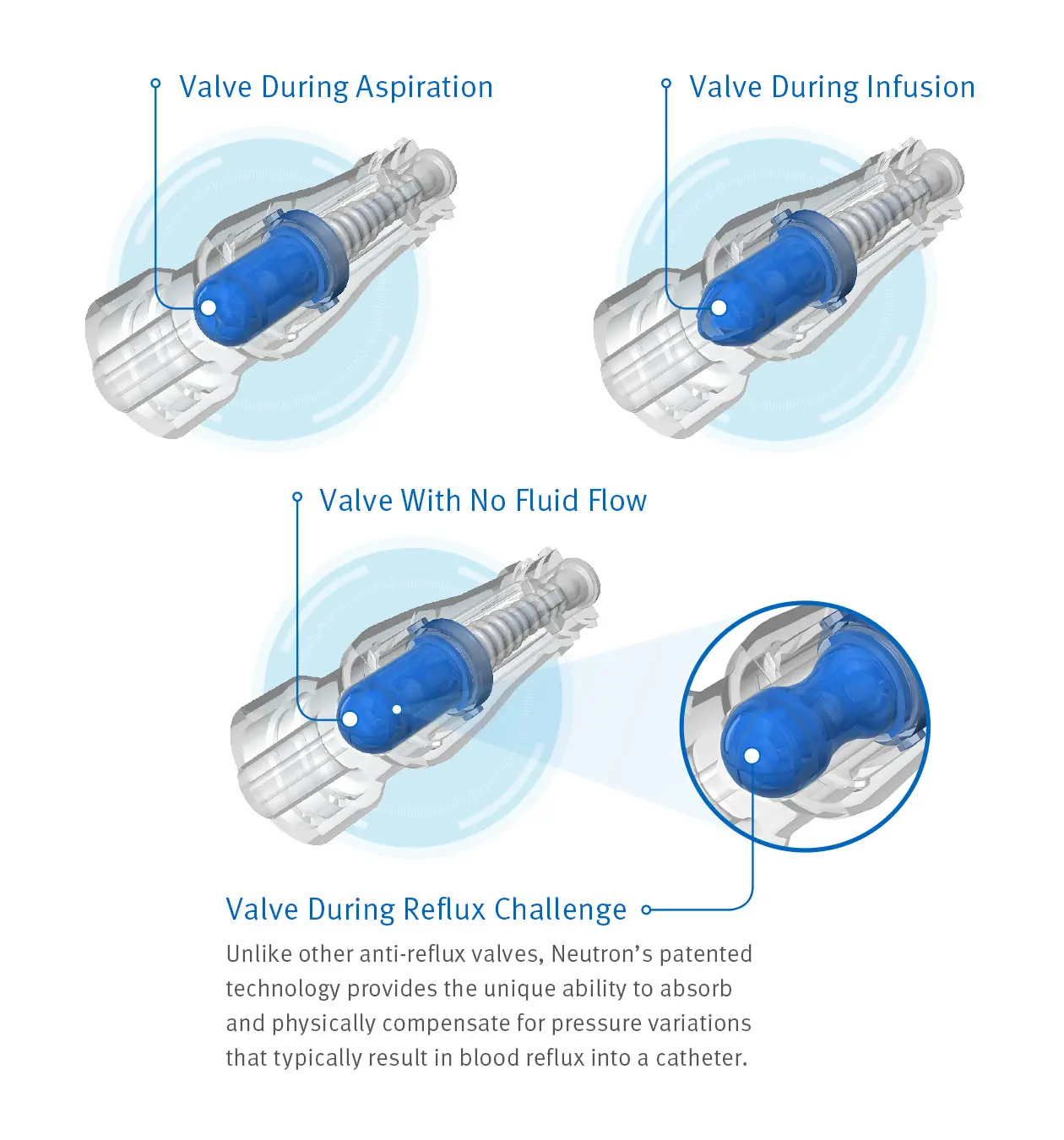
Clave Neutron™ Connector
Advanced Needlefree Anti-Reflux Technology
Maintaining catheter patency and minimizing occlusions can be important steps in your efforts to enhance patient safety and reduce costs. Clave Neutron connector combines clinically differentiated Clave infection control technology with a proprietary bi-directional silicone valve designed to prevent blood reflux and help minimize occlusions.
Clave Neutron connector is designed to help maintain catheter patency and prevent fluid displacement resulting from the four known causes of fluid displacement associated with needlefree connectors:
- Connection or disconnection of a luer
- Syringe plunger compression
- Patient vascular pressure changes (ie, coughing or sneezing)
- IV solution container run-dry, which may cause multiple forms of reflux into a catheter
After trialing the Clave Neutron connector, one pediatric oncology unit noted a 74.3% reduction in complete catheter occlusions and maintained a 32.1% reduction after full implementation.9

SuperCath® 5 – 26G Safety IV Catheter
Safety peripheral IV catheter technology designed to help access small, difficult vessels
Designed to optimize vessel entry in your most delicate patients, the SuperCath 5 is the first and only 26G straight safety catheter. This unique needle size and integrated needle notch helps you improve first-stick proficiency and avoid multiple insertion attempts by maximizing the speed and likelihood of flash visualization while accessing small, difficult vessels.
Helping deliver safe and effective care while complying with clinical recommendations
Infusion Therapy Standards of Practice recommendation: “Always select the smallest gauge peripheral catheter that will accommodate the prescribed therapy and patient need.”11

Optimize Supply Chain
Optimize the supply of your essential IV consumables
Standardizing on ICU Medical IV consumables gives you best-in-class Clave technology and access to our full portfolio of components to optimize your supply chain across dedicated and non-dedicated sets as well as the broadest offering of off-the-shelf IV sets tailored to a range of clinical needs.
Product Information
Related Products
Product Inquiry
Please enter your details into the following form.
References
SuperCath is a registered trademark of MediKit Co., Ltd.
-
Ryder, M., DeLancey-Pulcini, E., Parker, A., & James, G. (2023). Bacterial transfer and biofilm formation in needleless connectors in a clinically simulated in vitro catheter model. Infection Control & Hospital Epidemiology, 1-9. doi:10.1017/ice.2023.60
-
JD Brown, HA Moss, TSJ Elliott. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997.; 36:181-189.
-
Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med 2008;36: 2558–2561.
-
Bouza E, Munoz P, Lopez-Rodriguez J, et al. A needleless closed system device (Clave) protects from intravascular catheter tip and hub colonization: a prospective randomized study.
J Hosp Infect. 2003; 54:279-287.
-
Guidelines for the Prevention of Intravascular Catheter-Related Bloodstream Infections, 2011 (Updated Recommendations July 2017)
-
Breznock EM, DVM, PhD, Diplomate ACVS, Sylvia CJ, DVM, MS, BioSurg, Inc. The in vivo evaluation of the flushing efficiency of different designs of clear needlefree connectors, March 2011.
-
Data on file at ICU Medical. Low Volume Flush Characteristics of Unique Needlefree Connectors, M1-1223 Rev. 1.
-
Tanner J. Developing a Closed, Intravenous Medication System for a Neonatal Intensive Care Unit. Neonatal Intensive Care Journal, July 2012.
-
Observational In-Vivo Evaluation of the Neutron Needlefree Catheter Patency Device and Its Effects on Catheter Occlusions in a Home Care Setting, 2011.
-
Dayna Holt, MSN, RN, CRNI, CPN, VA-BC, Stephanie Lawrence, RN, BSN. The Influence of a Novel Needleless Valve on Central Venous Catheter Occlusions in Pediatric Patients. Journal of the Association For Vascular Access, Dec. 2015.
-
Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2021, 8th Edition.