Clave™ Neutron™
Anti-Reflux Neutral Displacement Needlefree Connector
A needlefree neutral displacement connector featuring ICU Medical’s clinically-differentiated Clave infection control technology with a bidirectional valve designed to prevent blood reflux and help minimize occlusions.
The Clave Neutron Needlefree Neutral Displacement Connector is Designed to Reduce Reflux to Help Minimize Occlusions
Maintaining catheter patency and minimizing occlusions can be important steps in your efforts to enhance patient safety and help reduce costs.
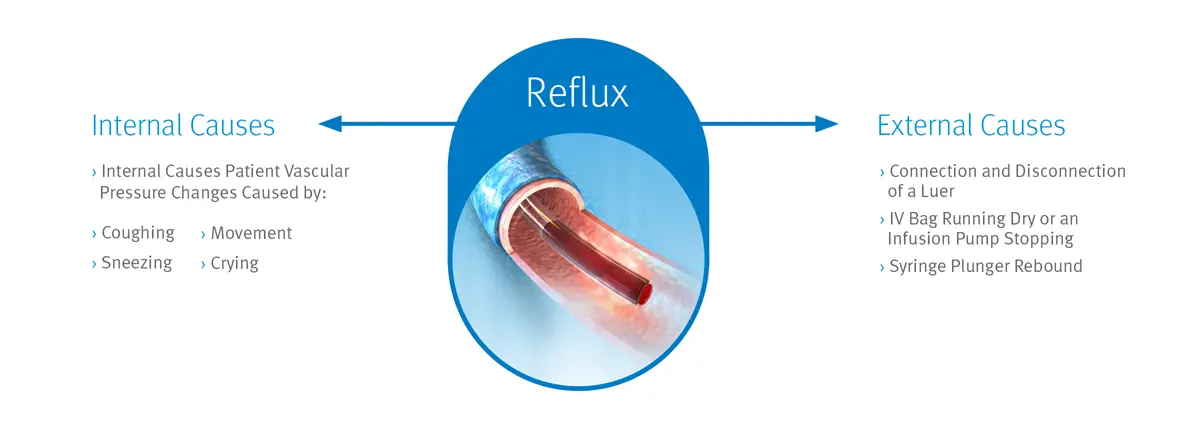
Despite your efforts, central line occlusions—which are frequently caused by blood reflux—remain a significant issue that can result in delays in critical patient care, increased risk of infection, and increased healthcare costs. That’s why reducing the risk of catheter occlusions may help you decrease the need for expensive declotting agents, such as tPA, and reduce the clinical costs associated with managing catheter occlusions.
Clave Neutron connector may help you reduce catheter occlusions by 50%8
Clave Neutron connectors innovative anti-reflux technology helps stop occlusions before they start while providing a safe and effective microbial barrier.
Our Neutron needlefree neutral displacement connector is designed to prevent fluid displacement resulting from the four known causes of displacement associated with needlefree connectors: connection or disconnection of a luer, syringe plunger compression, patient vascular pressure changes (e.g., coughing or sneezing), and IV solution container run-dry, which may cause multiple forms of reflux into a catheter.1 The Clave Neutron also utilizes ICU Medical’s Clave needlefree connector technology, which is proven to minimize contamination and help you lower the risk of catheter-related bloodstream infections (CRBSIs).2,3,4,5,6,7
Helping reduce catheter occlusions with the Clave Neutron needlefree neutral displacement connector may provide real-time clinical benefits.

Avoid Delays in Critical Patient Care
The Clave Neutron may help avoid delays in therapy of critical intravenous medications (e.g., antibiotics and oncolytics), nutritional support, and blood products.

Avoid Patient Discomfort and Pain
The Clave Neutron may help avoid patient discomfort and pain caused by unnecessary needlesticks, catheter restarts, and manipulation of the IV site.

Avoid Unnecessary Costs
The Clave Neutron may help minimize unnecessary costs that add up when treating an occlusion.

Help Reduce Risk of Infection
The Clave Neutron may help reduce the risk of infection by preventing thrombosis and minimizing IV line manipulation.
Designed to Prevent Fluid Displacement Resulting from the Four Known Causes of Displacement
Reflux of blood into the catheter has been shown to contribute to biofilm formation and catheter occlusion.
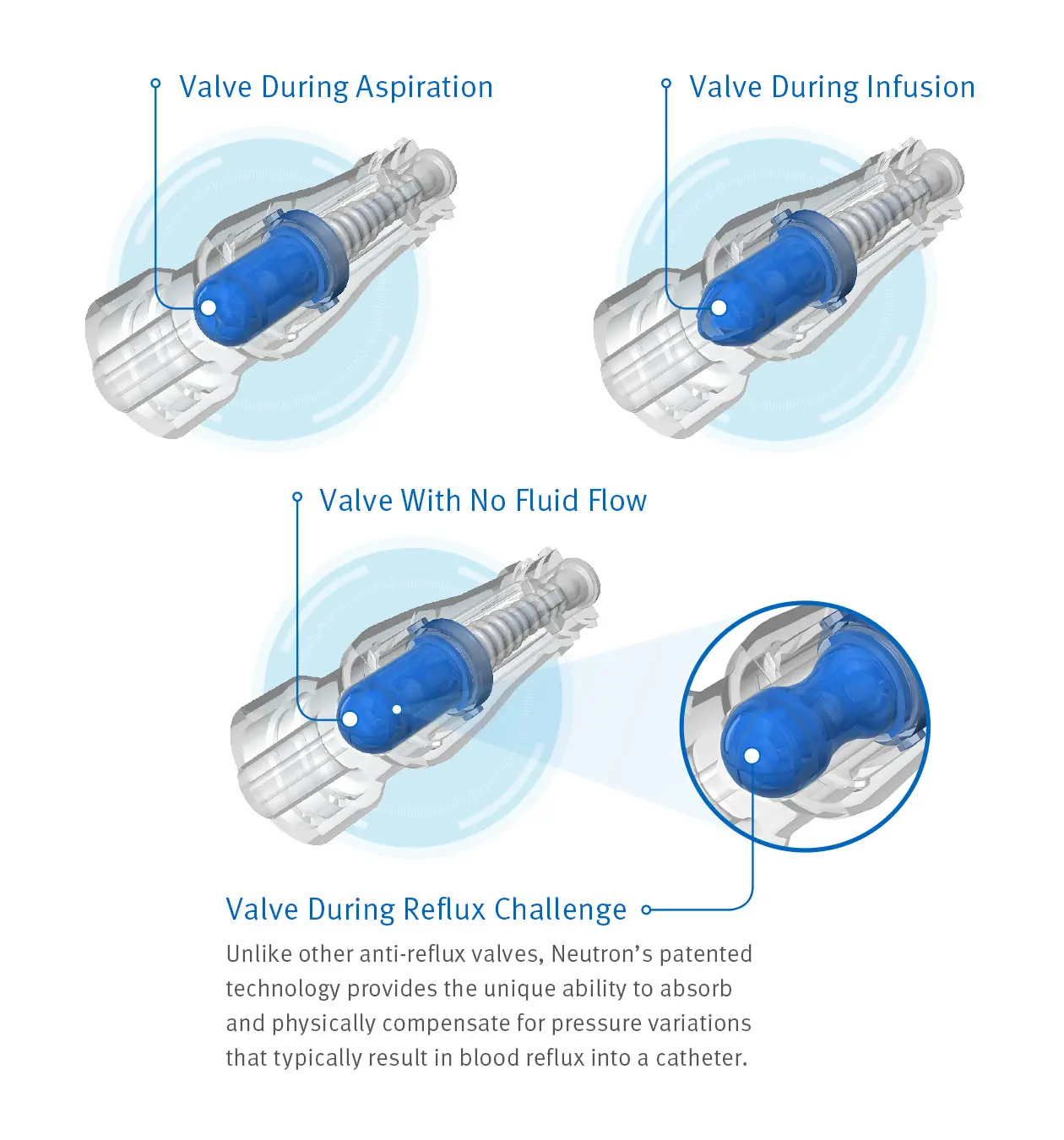
Advanced Anti-Reflux Technology
Because of an innovative design incorporating a proprietary, bidirectional silicone valve and bellows feature to help prevent reflux, Clave Neutron helps maintain catheter patency during the times traditional connectors have been shown to occlude most often.
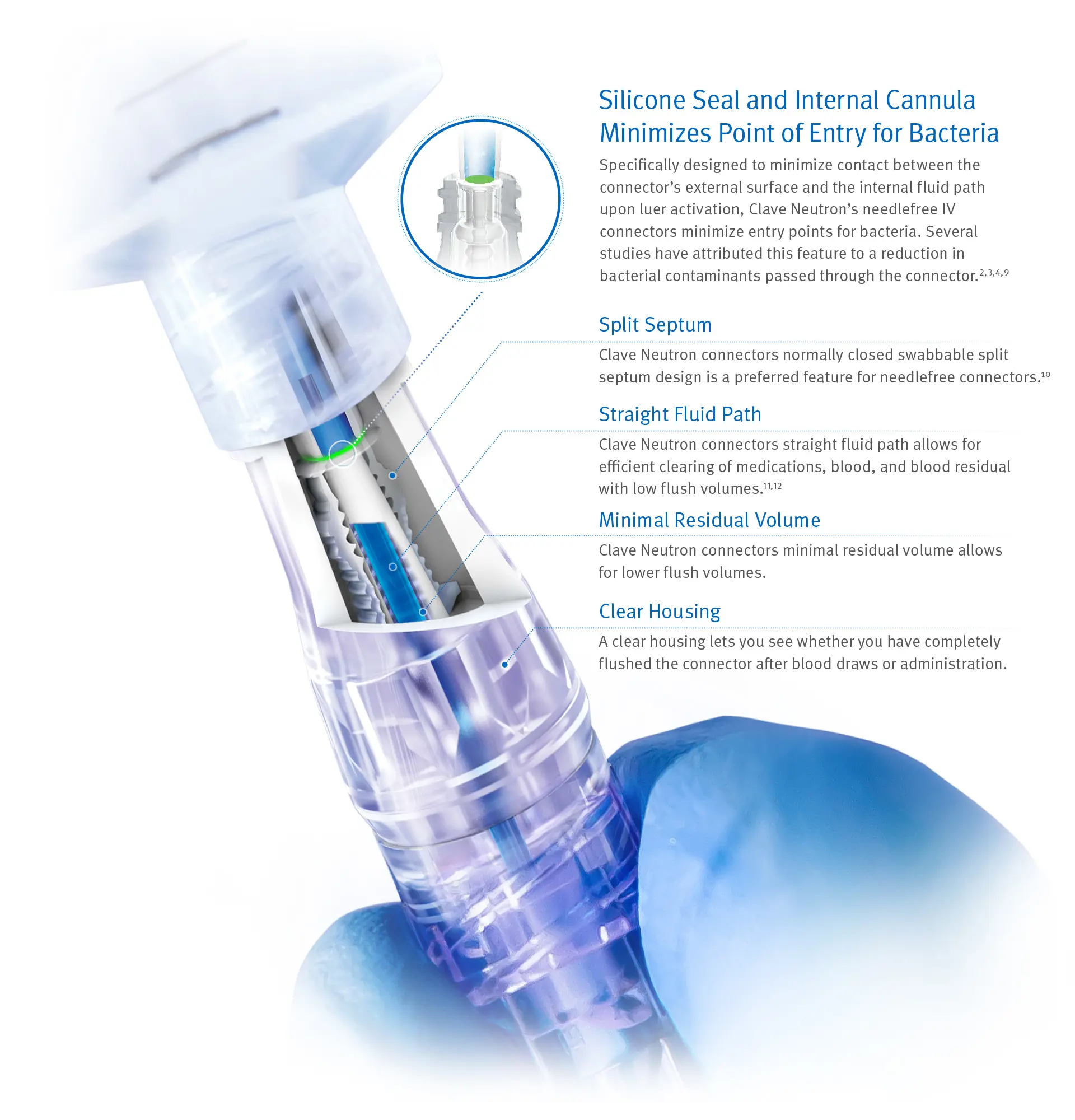
Clinically-differentiated Infection Control Technology Proven to Minimize Bacterial Contamination2,3,4,6,7
Clave Neutron can help your efforts to reduce bloodstream infections by minimizing entry points for bacteria and maximizing the effectiveness of every flush.
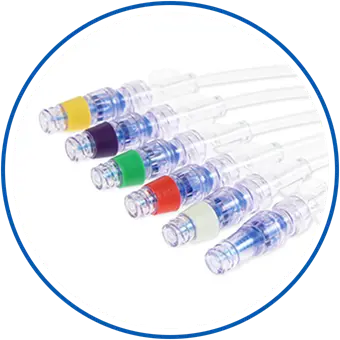
Add a Splash of Color for Quick and Easy Line Identification
Customize Neutron connector with a variety of color-coded rings to help you improve IV line management and avoid medication mix-ups.
Color-coded needlefree IV connector rings designed to help reinforce your facility's line-identification initiatives:
- Enhance patient safety and reduce the possibility of medication errors
- Quickly access the proper infusion port in emergency situations
- Improve connector change interval compliance with better needlefree connector identification
Technical Specifications
Drug Compatibility

Neutron’s saline flush option is designed to help you reduce risks, cost, and time associated with heparin use.
Warning: Clave connectors may be incompatible with some male-luer connectors including prefilled glass syringes. To avoid damage to the Clave or syringes or male luers which may result in delays of medication administration and possible serious adverse events, users should confirm mating luers or syringes have an internal diameter range of 0.062” to 0.110”. Check the internal diameter of the male-luer connector of the mating syringe prior to using it to access the Clave. Products outside of these dimensional tolerances should not be used.
Product Information
Related Products
Product Inquiry
Please enter your details into the following form.
References
- ICU Medical Clave Neutron 510(k) K100434, June 24, 2010
- Ryder M, RN, PhD. Comparison of Bacterial Transfer and Biofilm Formation on Intraluminal Catheter Surfaces Among Twenty Connectors in a Clinically Simulated In Vitro Model. Presented at World Congress Vascular Access (WoCova) 2018.
- JD Brown, HA Moss, TSJ Elliott. The potential for catheter microbial contamination from a needleless connector. J Hosp Infect. 1997.; 36:181-189.
- Yebenes J, Delgado M, Sauca G, Serra-Prat M, Solsona M, Almirall J, et al. Efficacy of three different valve systems of needlefree closed connectors in avoiding access of microorganisms to endovascular catheters after incorrect handling. Crit Care Med 2008;36: 2558–2561.
- Moore C, RN, MBA, CIC. Maintained Low Rate of Catheter-Related Bloodstream Infections (CR-BSIs) After Discontinuation of a Luer Access Device (LAD) at an Academic Medical Center. Poster presented at the annual Association for Professionals in Infection Control and Epidemiology (APIC) Conference 2010, Abstract 4-028.
- Data on file at ICU Medical. Microbial Ingress Study on Clave Technology Study commissioned by ICU and conducted by Alcami, 2016.
- Data on file at ICU Medical. Microbial Ingress Study on Neutron Connector. Study commissioned by ICU and conducted by Alcami, 2016.
- Observational In-Vivo Evaluation of the Neutron™ Needlefree Catheter Patency Device and its Effects on Catheter Occlusions in a Home Care Setting, 2011.
- Bouza E, Munoz P, Lopez-Rodriguez J, et al. A needleless closed system device (Clave™) protects from intravascular catheter tip and hub colonization: a prospective randomized study. J Hosp Infect. 2003; 54:279-287.
- Guidelines for the Prevention of Intravascular Catheter-Related Bloodstream Infections, 2011 (Updated Recommendations July 2017)
- Breznock EM, DVM, PhD, Diplomate ACVS, Sylvia CJ, DVM, MS, BioSurg, Inc. The in vivo evaluation of the flushing efficiency of different designs of clear needlefree connectors, March 2011.
- Data on file at ICU Medical. Low Volume Flush Characteristics of Unique Needlefree Connectors M1-1223 Rev. 1.




